Summary
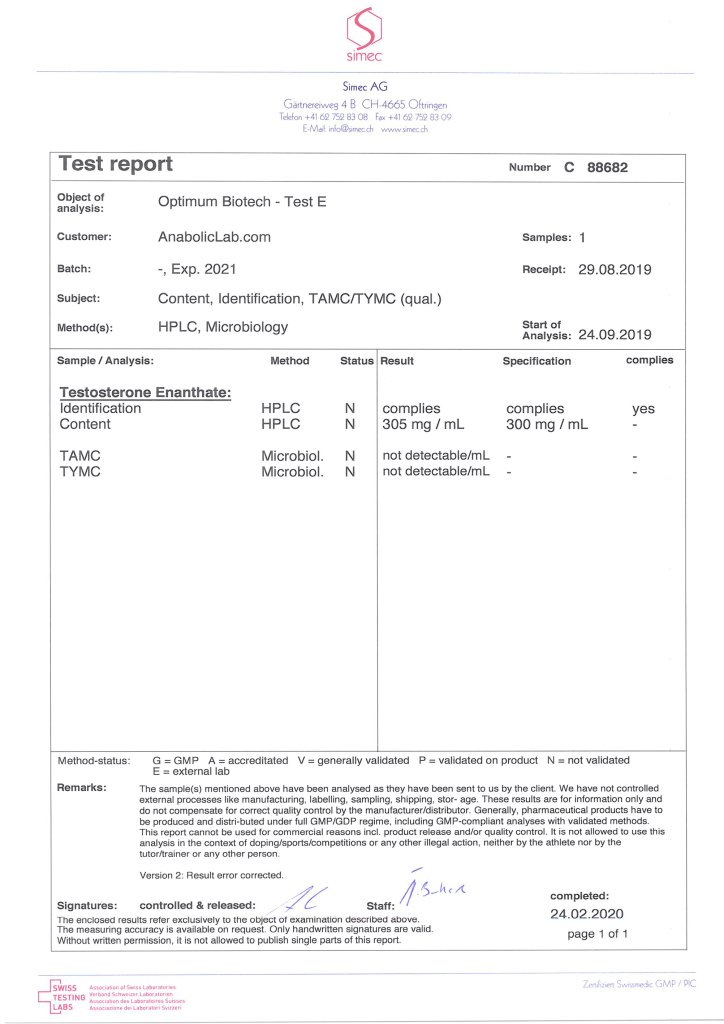
The product Testosterone Enanthate, tested under Optimum Biotech – Test E, underwent independent analysis to verify its quality and composition. The sample, with an expiry date of 2021, was submitted by AnabolicLab.com and analyzed by SIMEC AG, a laboratory specializing in pharmaceutical testing. The analysis confirmed that the product complies with its specifications, containing 305 mg/ml of Testosterone Enanthate, slightly exceeding the labeled claim of 300 mg/ml by 1.67%. Microbiological testing indicated no detectable contamination.
The testing process began on 24 September 2019, following receipt of the sample on 29 August 2019. While the results confirm accurate dosing and safety, it is important to note that these findings pertain to a single batch and should not be generalized to all batches. Independent third-party testing remains critical for comprehensive product verification. This report is shared as part of educational and harm reduction efforts.
Detailed Report
Product Overview
- Manufacturer: Optimum Biotech
- Product Name: Testosterone Enanthate
- Active Ingredient: Testosterone Enanthate
- Batch Number: Not provided
- Expiration Date: 2021
- Delivery Method: Injectable
Sample Acquisition and Testing
- Task Number: C88682
- Testing Ordered: 29 August 2019
- Sample Received: 29 August 2019
- Analysis Start Date: 24 September 2019
- Analysis Conducted By: SIMEC AG
- Product Submitted By: AnabolicLab.com
- Analysis Paid For By: AnabolicLab.com
Testing Results
- Specification: 300 mg/ml (as stated on the label)
- Measured Concentration: 305 mg/ml
- Accuracy: 101.67% (1.67% above the label claim)
Microbiological Contamination Testing:
- Total Aerobial Microbiological Count (TAMC): Not detectable/mL
- Total Yeast and Mold Count (TYMC): Not detectable/mL
Evaluation of Third-Party Testing
This analysis confirms that the product complies with its specifications and safety standards. However, it is critical to acknowledge that the results pertain only to the tested batch and may not reflect the consistency of other batches. Independent third-party testing of multiple batches is recommended to ensure reliability. While SIMEC AG is recognized for its transparency and rigorous testing, these findings should be interpreted within the context of batch-specific analysis.
Conclusion
The analysis confirms that Testosterone Enanthate meets its labeled claim, with a slight overdosage of 1.67%. Microbiological testing indicates no contamination, suggesting a high standard of safety and quality control for this batch. This report is provided as an educational resource to support harm reduction and informed decision-making in the anabolic steroid market.
Disclaimer
This report is published for educational and harm reduction purposes. The findings pertain solely to the tested batch and do not generalize to all products under the same name. Readers are encouraged to use this information responsibly and cross-reference results with independent third-party testing where possible.