Summary
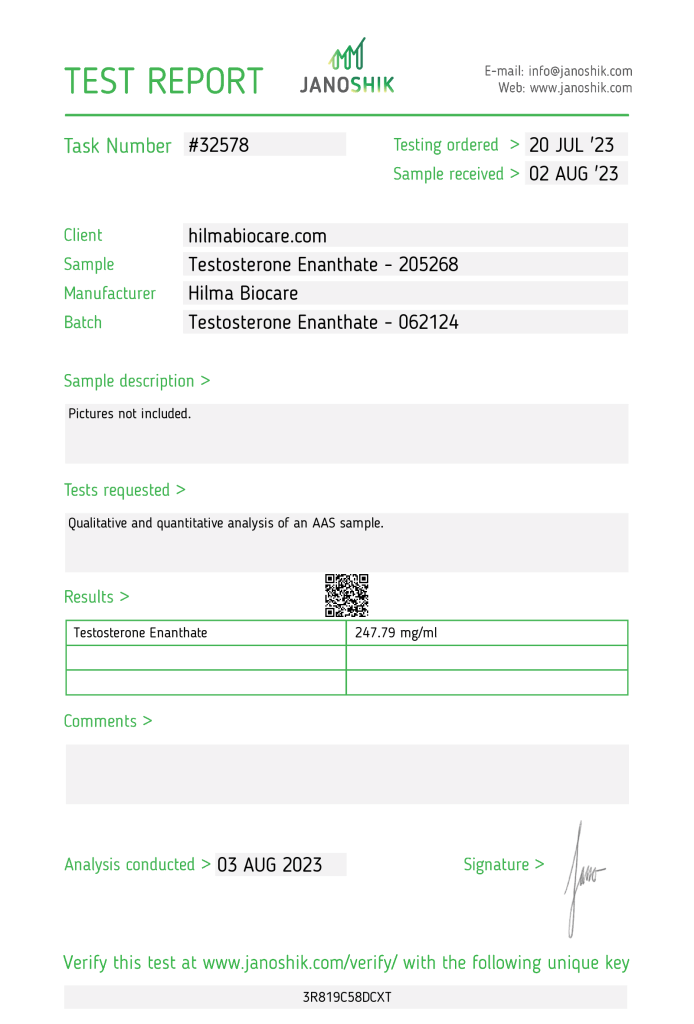
The product Testosterone Enanthate, manufactured by Hilma Biocare, underwent independent analysis to verify its quality and composition. The sample, procured and funded by hilmabiocare.com, a manufacturer, was analyzed by Janoshik Analytical, a laboratory specializing in pharmaceutical testing. The analysis confirmed the presence of Testosterone Enanthate, with a measured concentration of 247.79 mg/ml, slightly below the labeled claim of 250 mg/ml by 0.88%.
The testing process began on 20 July 2023, with the sample received on 2 August 2023, and analysis completed on 3 August 2023. As the sample was procured and paid for by the manufacturer, there is a potential for bias. Manufacturer-funded testing should be approached with caution as manufacturers may selectively submit optimal batches for testing. This report is shared as part of an ongoing commitment to harm reduction and informed decision-making.
Detailed Report
Product Overview
- Manufacturer: Hilma Biocare
- Product Name: Testosterone Enanthate
- Active Ingredient: Testosterone Enanthate
- Batch Number: Testosterone Enanthate-062124
- Expiration Date: Not provided
- Delivery Method: Injectable
Sample Acquisition and Testing
- Task Number: #32578
- Testing Ordered: 20 July 2023
- Sample Received: 2 August 2023
- Analysis Conducted By: Janoshik Analytical
- Product Submitted By: hilmabiocare.com (Manufacturer)
- Analysis Paid For By: hilmabiocare.com
Testing Results
- Specification: 250 mg/ml (as stated on the label)
- Measured Concentration: 247.79 mg/ml
- Accuracy: 99.12% (0.88% below the label claim)
Verification Details
- Verification URL: https://janoshik.com/tests/32578_3R819C58DCXT
- Originally Published: https://thinksteroids.com/community/posts/3221180/
Evaluation of Manufacturer-Submitted Testing
This analysis demonstrates the accuracy of the tested product but requires careful consideration due to its submission and funding by the manufacturer hilmabiocare.com. Manufacturer-funded testing may introduce potential bias as manufacturers often select batches known to meet quality standards. These results should be cross-referenced with independent or third-party testing to ensure consistency across market-available batches. Independent validation of additional batches would strengthen consumer confidence and reliability.
Conclusion
The analysis confirms that Testosterone Enanthate slightly underdoses its labeled claim, measured at 247.79 mg/ml. While this reflects relatively strong quality control for the tested batch, the manufacturer-funded nature of the testing highlights the need for transparency and independent corroboration. This report is shared as part of an ongoing commitment to harm reduction and consumer education within the anabolic steroid market.
Disclaimer
This report is published for educational and harm reduction purposes. Manufacturer-funded testing, while potentially useful, may involve inherent biases. The findings pertain solely to the tested batch and do not generalize to all products under the same name. Readers are encouraged to cross-reference these results with independent third-party testing and use this information responsibly.