Introduction
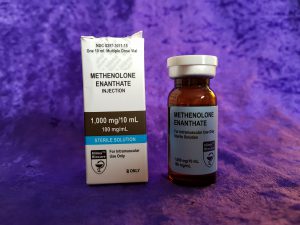
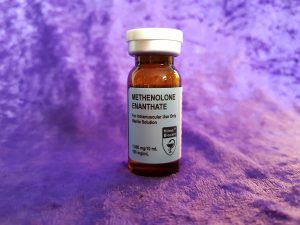
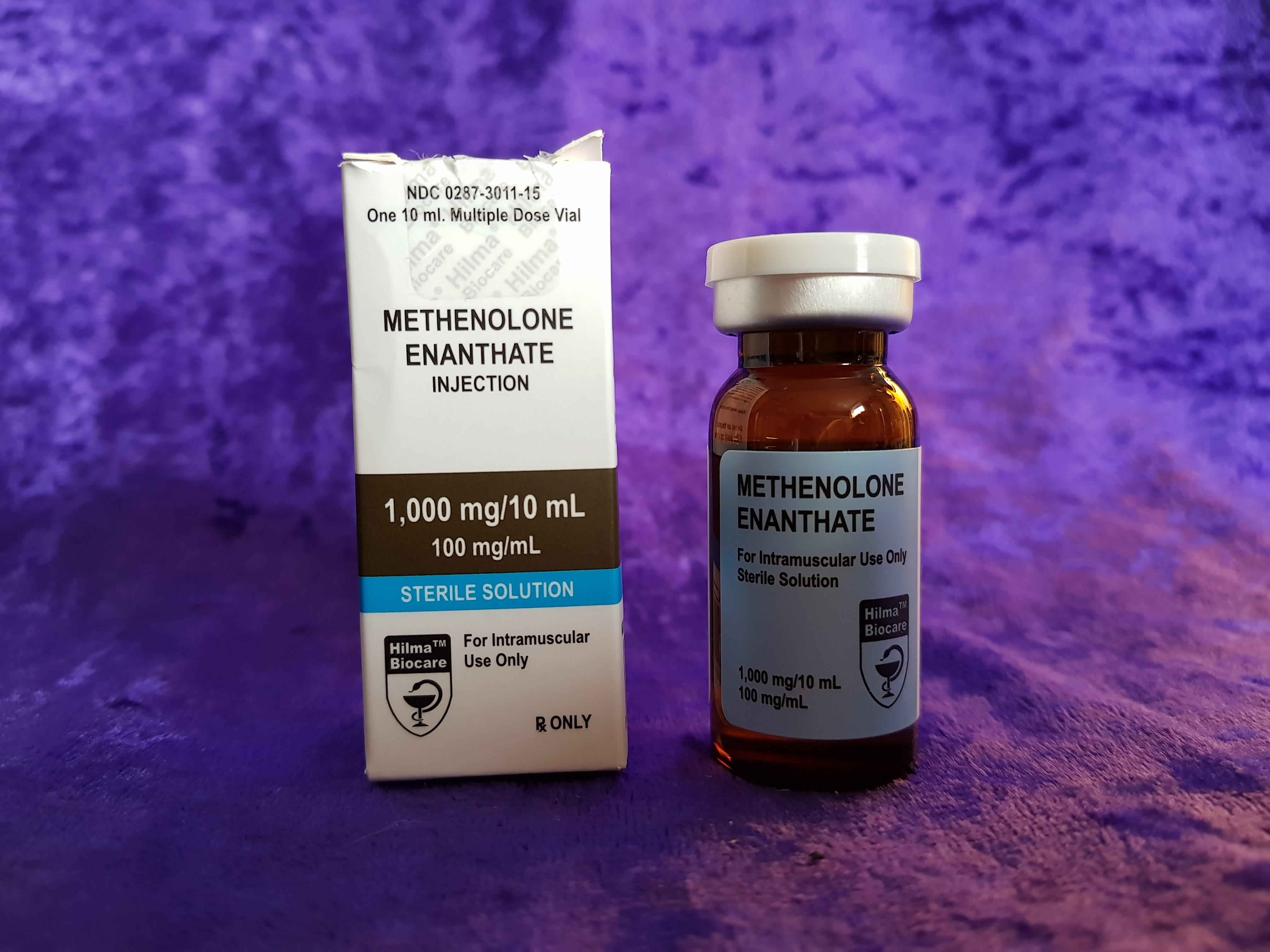
Hilma BioCare Methenolone Enanthate is presented in a 10-milliliter multidose vial, labeled to contain 100 milligrams of methenolone enanthate per milliliter. To verify the manufacturer’s claims and evaluate its quality, AnabolicLab procured samples of this product from a European-based internet source. These samples were purchased between January 1, 2018, and February 28, 2018, and submitted to SIMEC AG, a globally recognized and accredited analytical laboratory, for comprehensive testing.
The analysis, fully funded by AnabolicLab, included HPLC-UV quantitative dosage testing, as well as microbiological testing to ensure safety. By publishing these results, AnabolicLab provides consumers with transparent, reliable information about anabolic steroid products, supporting harm reduction efforts and promoting accountability within the market.
Testing Results and Authentication
The samples were received by SIMEC AG on February 28, 2018, and the report was finalized on March 14, 2018.
Product Overview
- Manufacturer: Hilma BioCare
- Product Name: Methenolone Enanthate
- Active Ingredient: Methenolone Enanthate
- Batch Number: 037962
- Expiration Date: January 2020
- Delivery Method: Injectable (10-milliliter multidose vial)
- Specification: 100 mg/ml methenolone enanthate
Sample Acquisition and Testing
- Date Purchased: Between January 1, 2018, and February 28, 2018
- Source: European-based internet supplier
- Product Submitted By: AnabolicLab
- Analysis Paid For By: AnabolicLab
- Analysis Performed By: SIMEC AG
- Date Received by Lab: February 28, 2018
- Date Report Completed: March 14, 2018
Testing Results
Quantitative Dosage Testing (HPLC-UV):
- Claimed Dosage: 100 mg/ml methenolone enanthate
- Measured Dosage: 99.5 mg/ml methenolone enanthate
- Accuracy: 99.5% (very close to label claim)
- Variance: -0.5% (measured dosage was 0.5% below the label claim)
Microbiological Contamination Testing:
- Total Aerobial Microbiological Count (TAMC): No contamination detected
- Total Yeast and Mold Count (TYMC): No contamination detected
Summary
The analysis of Hilma BioCare Methenolone Enanthate confirmed its authenticity and adherence to its label claims. The measured content of 99.5 mg/ml methenolone enanthate demonstrated excellent consistency, with a minimal variance of -0.5% from the label claim. Microbiological testing revealed no contamination, confirming the product’s safety. These results highlight the product’s reliability and reinforce the importance of independent testing in promoting transparency in the anabolic steroid market.
Commitment to Harm Reduction
AnabolicLab’s harm reduction initiative focuses on providing consumers with accurate and independently verified information about anabolic steroid products. By publishing comprehensive test results, AnabolicLab helps consumers make informed decisions, supports safety, and encourages greater accountability among manufacturers.
Disclaimer
The information provided here is for educational and harm reduction purposes only. AnabolicLab does not endorse or promote the use of anabolic steroids or other controlled substances.
Discuss the Hilma BioCare Methenolone Enanthate lab test results on the AnabolicLab Forum.

(The following images are photographs of the actual product that was submitted for testing.)