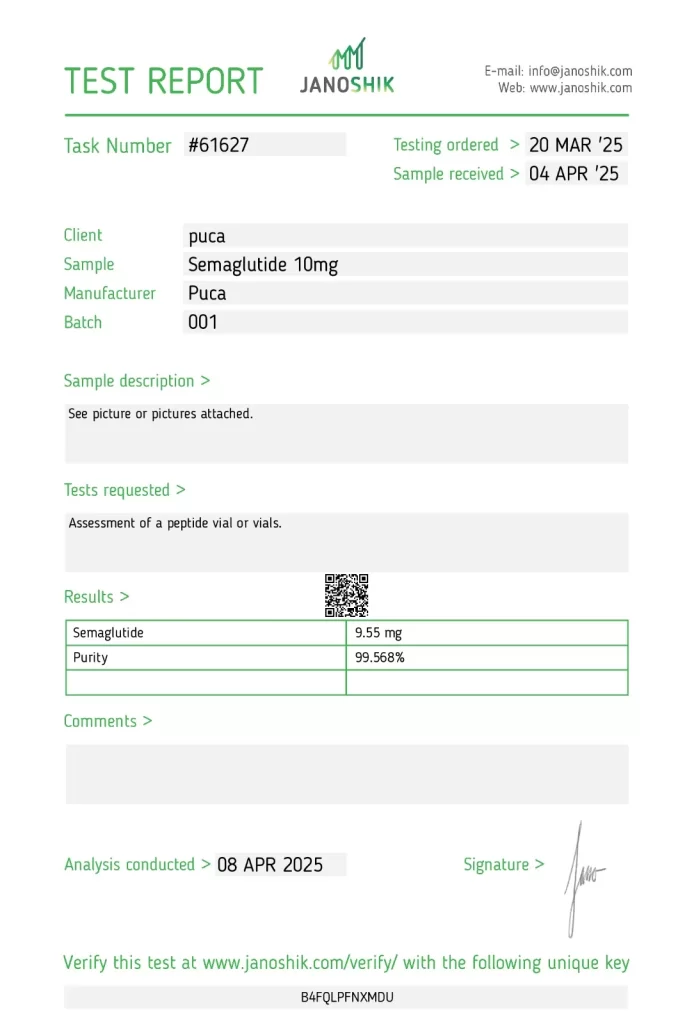
A sample of Semaglutide 10mg, manufactured by PUCA, was submitted by the manufacturer and tested to assess active ingredient content and purity. The test was conducted by Janoshik Analytical, a third-party lab known for its rigorous peptide verification procedures.
The results showed 9.55 mg of Semaglutide with a 99.568% purity, indicating a 4.5% underdose from the label claim. While the peptide quality remains excellent, the slight discrepancy in content should be noted for clinical and research users seeking precise dosing.
Detailed Report
Product Overview
- Manufacturer: PUCA
- Product Name: Semaglutide
- Active Ingredient: Semaglutide
- Labeled Concentration: 10 mg
- Measured Concentration: 9.55 mg
- Purity: 99.568%
- Batch Number: 001
- Expiration Date: Not Provided
- Delivery Method: Peptide vial
Sample Acquisition and Testing
- Task Number: #61627
- Testing Ordered: 20 March 2025
- Sample Received: 4 April 2025
- Analysis Conducted By: Janoshik Analytical
- Product Submitted By: PUCA (Manufacturer)
- Analysis Paid For By: PUCA (Manufacturer)
Testing Results
| Component/Test | Specification (Label Claim) | Measured Value | Accuracy | Variance |
|---|---|---|---|---|
| Semaglutide | 10 mg | 9.55 mg | 95.5% | -4.5% |
| Purity | — | 99.568% | — | — |
Verification Details
- Verification URL: https://janoshik.com/tests/61627_B4FQLPFNXMDU
Evaluation of Manufacturer Testing
The PUCA Semaglutide sample demonstrated strong purity at 99.568%, with a slight underdose of 4.5% relative to the labeled 10 mg amount. This falls within tolerable pharmaceutical margins but should still be taken into account for applications where precise dosing is essential.
As the sample was both submitted and funded by the manufacturer, it provides insight into PUCA’s internal quality practices. For full transparency, third-party or user-submitted test data should supplement this type of manufacturer-conducted testing.
Conclusion
The Janoshik-verified test of PUCA Semaglutide 10mg indicates a measured content of 9.55 mg and a high purity level of 99.568%. These results confirm a well-formulated peptide product with a minor deviation from the label, likely acceptable for most non-clinical uses.
Independent validation across additional lots is recommended to assess batch-to-batch consistency and reinforce PUCA’s quality claims.
Disclaimer
This report is intended for harm reduction and educational purposes. While conducted by a reliable lab, this test was manufacturer-submitted and may not reflect broader retail consistency. Independent testing remains key to ensuring quality and dosage integrity across product batches.