Summary
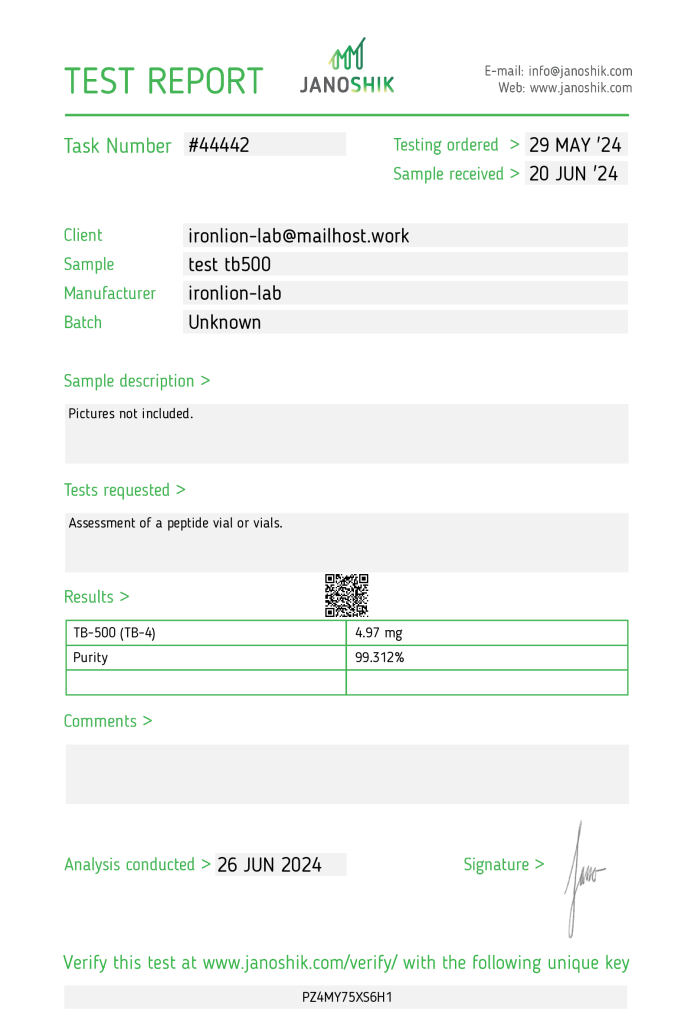
The product test tb500, manufactured by ironlion-lab, underwent independent testing to verify its authenticity and potency. The sample, with an unknown batch number, was submitted by ironlion-lab and analyzed by Janoshik Analytical, a laboratory specializing in pharmaceutical quality control. The analysis measured the presence of TB-500 (TB-4), with a concentration of 4.97 mg, which is 0.6% below the labeled claim of 5 mg per vial. The product’s purity was determined to be 99.312%.
The testing process began on 29 May 2024, with the sample received on 20 June 2024, and analysis completed on 26 June 2024. While the results confirm high purity and accurate dosing, scrutiny of manufacturer-submitted samples remains essential to ensure reliability. Independent third-party testing remains critical to validate these findings comprehensively. This report serves as an educational resource to promote harm reduction and informed decision-making.
Detailed Report
Product Overview
- Manufacturer: ironlion-lab
- Product Name: test tb500
- Active Ingredient: TB-500 (TB-4)
- Batch Number: Unknown
- Expiration Date: Not provided
- Delivery Method: Viral Powder
Sample Acquisition and Testing
- Task Number: #44442
- Testing Ordered: 29 May 2024
- Sample Received: 20 June 2024
- Analysis Conducted By: Janoshik Analytical
- Product Submitted By: ironlion-lab (Manufacturer)
- Analysis Paid For By: ironlion-lab (Manufacturer)
Testing Results
- Specification: 5 mg (as stated on the label)
- Measured Concentration: 4.97 mg
- Accuracy: 99.4% (0.6% below the label claim)
- Purity: 99.312%
Verification Details
Verification URL: https://janoshik.com/tests/44442_PZ4MY75XS6H1
Evaluation of Manufacturer-Submitted Testing
This analysis highlights that test tb500 contains TB-500 (TB-4) at a high purity level of 99.312%, with a measured amount of 4.97 mg per vial, closely aligning with the labeled 5 mg specification. The minor deviation in concentration is within an acceptable range. However, since the test was submitted and funded by ironlion-lab, careful evaluation is necessary to assess product consistency across different batches. While Janoshik Analytical is recognized for its transparency and rigorous testing protocols, these findings would benefit from independent validation through third-party testing across multiple samples.
Conclusion
The analysis confirms that test tb500 contains 4.97 mg of TB-500 (TB-4) per vial, which is 0.6% below the labeled claim. The high purity (99.312%) ensures product quality, and the minor deviation in concentration falls within an acceptable range. This batch exhibits strong quality control, but further independent third-party testing is encouraged to validate consistency across different batches. This report is provided to support educational and harm reduction efforts, helping consumers make informed choices regarding pharmaceutical and peptide products.
Disclaimer
This report is published for educational and harm reduction purposes. Manufacturer-submitted testing may involve inherent biases; however, it can still provide useful data when critically assessed alongside third-party or independent results. Readers are encouraged to use this information responsibly.