Summary
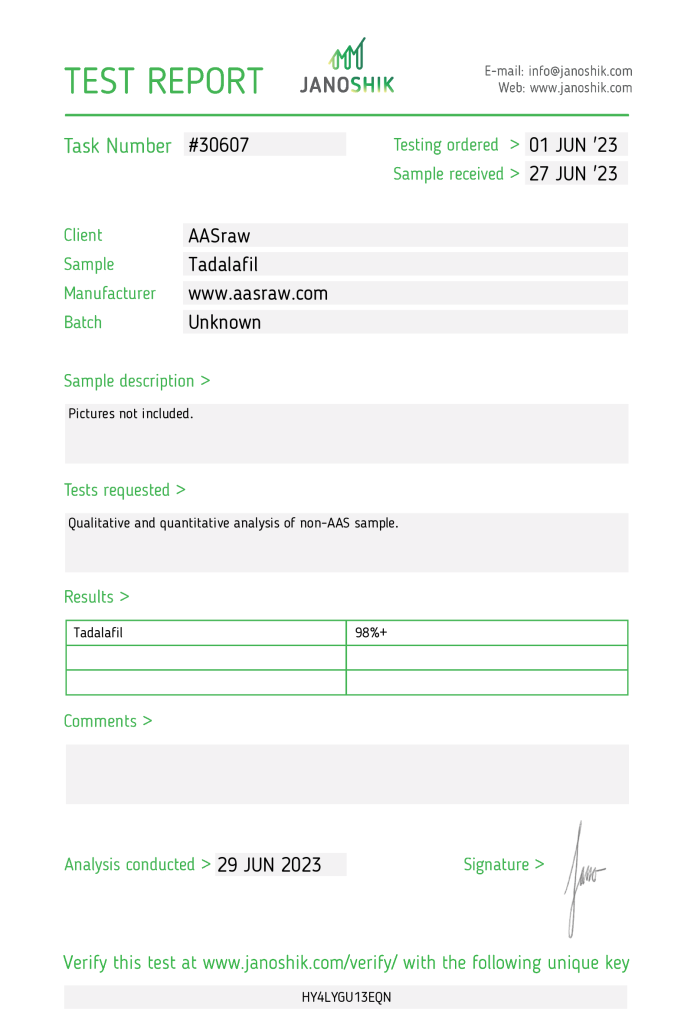
The product Tadalafil, manufactured by AAS Raw, underwent independent testing to confirm its composition and purity. The sample, identified by task number #30607, was submitted by AAS raw and analyzed by Janoshik Analytical, a laboratory specializing in pharmaceutical quality control. The analysis confirmed the presence of Tadalafil with a purity of 98%+.
The testing process began on 1 June 2023, with the sample received on 27 June 2023, and the analysis completed on 29 June 2023. While the results confirm a high level of purity, it is essential to consider that manufacturer-submitted samples may not represent the consistency of all products available on the market. Independent third-party testing remains crucial for comprehensive validation. This report is provided as an educational resource to promote harm reduction and informed decision-making.
Detailed Report
Product Overview
- Manufacturer: AAS Raw
- Product Name: Tadalafil
- Active Ingredient: Tadalafil
- Batch Number: Unknown
- Expiration Date: Not provided
- Delivery Method: Raw Powder
Testing Details
- Task Number: #30607
- Testing Ordered: 1 June 2023
- Sample Received: 27 June 2023
- Analysis Conducted By: Janoshik Analytical
- Product Submitted By: AAS Raw (Manufacturer)
- Analysis Paid For By: AAS Raw (Manufacturer)
Testing Results
- Specification: 100 mg
- Measured Purity: 98%+
Verification Details
- Verification URL: https://janoshik.com/tests/30607_HY4LYGU13EQN
Evaluation of Manufacturer-Submitted Testing
This analysis confirms that Tadalafil meets a high-purity standard of 98%+, but it is crucial to recognize that the sample was submitted and funded by AAS Raw. Manufacturers often select their highest-quality batches for testing, which may not accurately reflect the consistency of commercial batches. While Janoshik Analytical is known for transparent testing, further independent verification across multiple batches is advised for a more comprehensive assessment.
Conclusion
The test results indicate that Tadalafil from AAS Raw is of high purity (98%+). However, further independent testing is recommended to validate batch-to-batch consistency. This report serves as an educational resource for harm reduction and informed decision-making regarding pharmaceutical products.
Disclaimer
This report is published for educational purposes. Manufacturer-submitted testing may have inherent biases; however, it provides useful data when assessed alongside independent third-party results. Readers are encouraged to interpret this information responsibly.