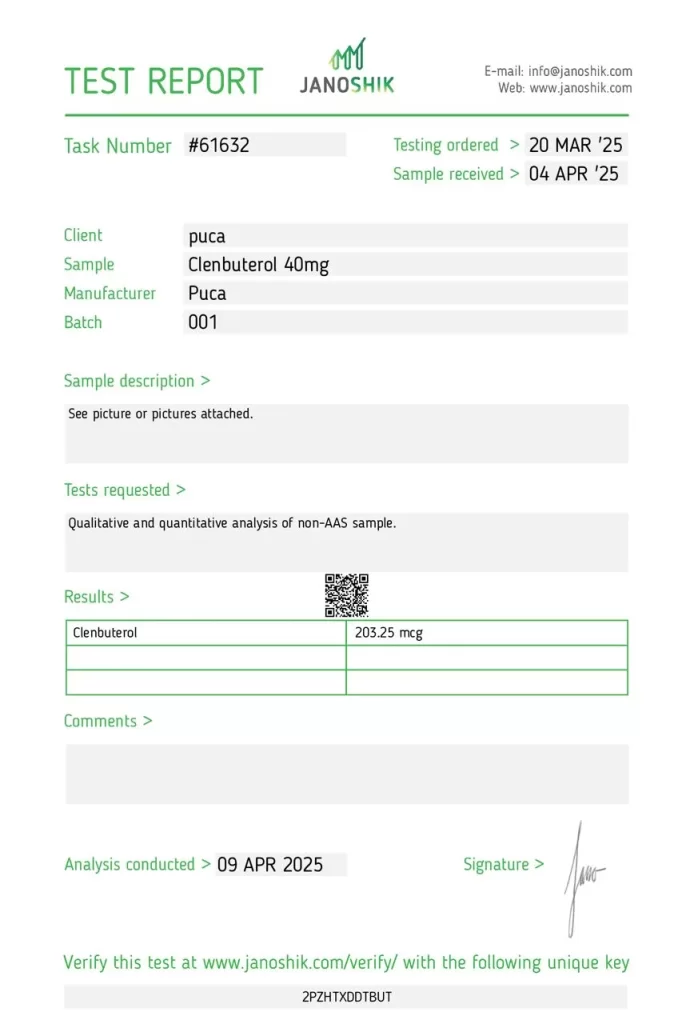
A sample of Clenbuterol (labeled 40mcg), manufactured by PUCA, was submitted by the manufacturer and tested to verify its actual dosage. The analysis was funded by PUCA, and testing was performed by Janoshik Analytical, an independent pharmaceutical quality control lab.
The result revealed a measured concentration of 203.25 mcg, which constitutes a 408.13% overdose compared to the label claim of 40 mcg. This magnitude of deviation presents a potential safety concern due to the drug’s potent adrenergic effects even at therapeutic doses.
Detailed Report
Product Overview
- Manufacturer: PUCA
- Product Name: Clenbuterol
- Active Ingredient: Clenbuterol
- Labeled Concentration: 40 mcg
- Measured Amount: 203.25 mcg
- Batch Number: 001
- Expiration Date: Not Provided
- Delivery Method: Non-AAS compound (presumed liquid vial)
Sample Acquisition and Testing
- Task Number: #61632
- Testing Ordered: 20 March 2025
- Sample Received: 4 April 2025
- Analysis Conducted By: Janoshik Analytical
- Product Submitted By: PUCA (Manufacturer)
- Analysis Paid For By: PUCA (Manufacturer)
Testing Results
| Component/Test | Specification (Label Claim) | Measured Value | Accuracy | Variance |
|---|---|---|---|---|
| Clenbuterol | 40 mcg | 203.25 mcg | 508.13% | +408.13% |
Verification Details
- Verification URL: https://janoshik.com/tests/61632_2PZHTXDDTBUT
Evaluation of Manufacturer Testing
The analysis of PUCA’s Clenbuterol sample revealed a massive overdose of over 400% compared to the label claim. While accurate chemical identification is confirmed, the extreme discrepancy in dosing may pose a significant health and safety risk, particularly for those using it as a sympathomimetic agent for fat loss or respiratory applications.
As this sample was submitted and funded by the manufacturer, further independent tests are recommended to rule out labelling or filling inconsistencies and ensure this finding isn’t isolated.
Conclusion
The Janoshik-verified test of PUCA Clenbuterol 40mcg indicates a severe overdose, with the sample testing at 203.25 mcg — 508.13% of the intended dose. Such a result, especially for a stimulant compound with a narrow therapeutic window, raises serious concerns regarding dosing accuracy and consumer safety.
Independent verification across additional batches is strongly advised to assess whether this finding reflects systemic manufacturing issues.
Disclaimer
This report is intended solely for educational and harm reduction purposes. The data represents a single batch tested by a reputable lab but submitted and funded by the manufacturer. Consumers are urged to seek third-party testing data and exercise extreme caution when dealing with substances that exhibit high potency at low dosages.