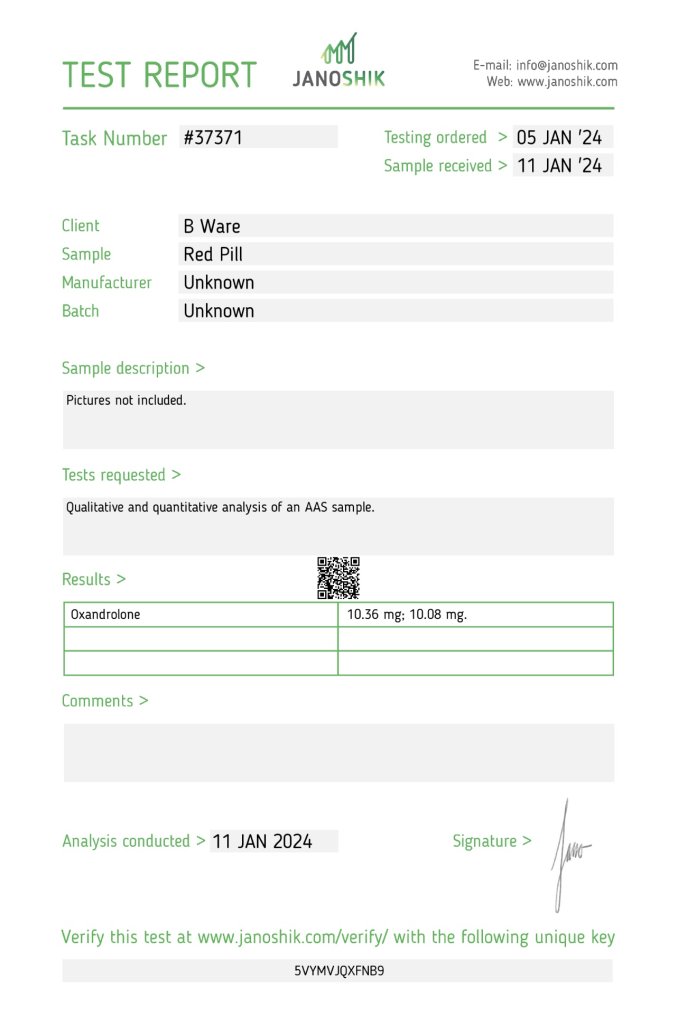
The product Oxandrolone (10 mg per tablet), manufactured by Optitropin, was submitted for independent testing by individual B Ware. The sample was analyzed by Janoshik Analytical to verify its potency. The results indicate that the sample contained 10.36 mg and 10.08 mg of Oxandrolone per tablet in separate tests, which represents 103.6% and 100.8%, respectively, of the labeled claim of 10 mg.
The Average Measured Dosage was calculated at 10.22 mg, which equates to 102.2% of the labeled claim, indicating a slight overage within pharmaceutical tolerances. Additionally, the Relative Standard Deviation (RSD%) was determined to be 1.94%, demonstrating minimal variation between tablets. Since the RSD% is well below the 6% compliance threshold, the batch meets pharmaceutical standards for uniformity.
Testing was initiated on 5 January 2024, with the sample received on 11 January 2024, and the analysis conducted on the same day. The measured concentrations are slightly above the labeled dose but remain within an acceptable pharmaceutical variance, confirming strong quality control for this batch.
This report is published to promote transparency and harm reduction, helping consumers make informed decisions regarding pharmaceutical product use.
Detailed Report
Product Overview
- Manufacturer: Optitropin
- Product Name: Oxandrolone 10
- Active Ingredient: Oxandrolone
- Batch Number: Unknown
- Expiration Date: Not provided
- Delivery Method: Oral Tablet
Sample Acquisition and Testing
- Task Number: #37371
- Testing Ordered: 5 January 2024
- Sample Received: 11 January 2024
- Analysis Conducted By: Janoshik Analytical
- Product Submitted By: B Ware (Individual)
- Analysis Paid For By: B Ware (Individual)
Testing Results
| Specification | Measured Concentration | Accuracy | Variance |
|---|---|---|---|
| 10 mg (as per label) | 10.36 mg | 103.6% | +3.6% |
| 10 mg (as per label) | 10.08 mg | 100.8% | +0.8% |
- Average Measured Dosage:10.22 mg
- Relative Standard Deviation (RSD %):1.94%
- Compliance with RSD%: ✅ Compliant (≤6%)
Verification Details
- Verification URL: https://janoshik.com/tests/37371_5VYMVJQXFN89
Evaluation of Individual-Submitted Testing
The analysis confirms that the Oxandrolone (10 mg per tablet) sample meets and slightly exceeds its labeled claim in separate tests (103.6% and 100.8% of the label claim). The Relative Standard Deviation (RSD%) of 1.94% indicates excellent uniformity between tested tablets, reinforcing high product consistency.
Since the RSD% is significantly below the 6% compliance threshold, these results suggest strong manufacturing control over ingredient distribution. This level of consistency ensures that dosage variations are minimal, reducing potential risks associated with fluctuating potency.
As the sample was submitted by an independent consumer (B Ware) with no commercial affiliations, there is no potential bias from reseller or manufacturer submissions. However, as with any single-batch test, additional independent testing across multiple batches is advised to validate product consistency over time.
Conclusion
The results confirm that Optitropin’s Oxandrolone (10 mg) met or slightly exceeded its labeled potency, with measured concentrations of 10.36 mg and 10.08 mg per tablet. The Average Measured Dosage of 10.22 mg represents 102.2% accuracy of the labeled claim, while the Relative Standard Deviation (RSD%) of 1.94% indicates strong dosage uniformity across tablets.
This minor overage is well within standard pharmaceutical tolerances, suggesting high-quality control for this batch.
Consumers are encouraged to continue verifying product quality through independent third-party testing, as this remains a crucial step in harm reduction and informed decision-making.
Disclaimer
This report is for educational and harm reduction purposes only. The results pertain solely to the batch tested and may not reflect the quality of other batches. Although B Ware has no commercial interests, readers are encouraged to critically assess this information alongside third-party or independent data to form a comprehensive understanding of product consistency. Always consult healthcare professionals and adhere to local regulations before using such products.m reduction purposes only. The results pertain solely to the batch tested and may not reflect the quality of other batches. Although B Ware has no commercial interests, readers are encouraged to critically assess this information alongside third-party or independent data to form a comprehensive understanding of product consistency. Always consult healthcare professionals and adhere to local regulations before using such products.