Summary
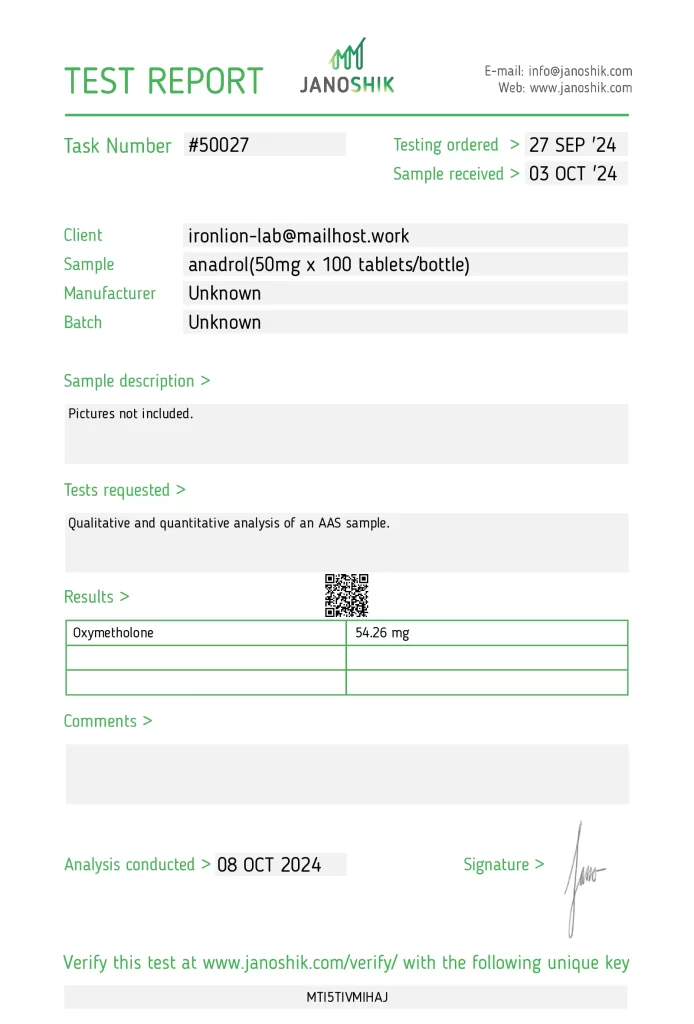
The product anadrol (50mg x 100 tablets/bottle), with an ironlion-lab manufacturer, underwent independent testing to verify its authenticity and potency. The sample, with an unknown batch number, was submitted by ironlion-lab and analyzed by Janoshik Analytical, a laboratory specializing in pharmaceutical quality control. The analysis measured the presence of Oxymetholone, with a concentration of 54.26 mg, which is 8.52% above the labeled claim of 50 mg per tablet.
The testing process began on 27 September 2024, with the sample received on 3 October 2024, and analysis completed on 8 October 2024. While the results confirm the presence of Oxymetholone, the higher-than-expected concentration raises concerns regarding dosing accuracy. Scrutiny of manufacturer-submitted samples remains essential to ensure reliability. Independent third-party testing remains critical to validate these findings comprehensively. This report serves as an educational resource to promote harm reduction and informed decision-making.
Detailed Report
Product Overview
- Manufacturer: ironlion-lab
- Product Name: anadrol (50mg x 100 tablets/bottle)
- Active Ingredient: Oxymetholone
- Batch Number: Unknown
- Expiration Date: Not provided
- Delivery Method: Oral (tablet)
Sample Acquisition and Testing
- Task Number: #50027
- Testing Ordered: 27 September 2024
- Sample Received: 3 October 2024
- Analysis Conducted By: Janoshik Analytical
- Product Submitted By: ironlion-lab (Manufacturer)
- Analysis Paid For By: ironlion-lab (Manufacturer)
Testing Results
- Specification: 50 mg (as stated on the label)
- Measured Concentration: 54.26 mg
- Accuracy: 108.52% (8.52% above the label claim)
Verification Details
Verification URL: https://janoshik.com/tests/50027_MTI5TIVMIHAJ
Evaluation of Manufacturer-Submitted Testing
This analysis highlights that anadrol (50mg x 100 tablets/bottle) contains Oxymetholone at a higher concentration than labeled (54.26 mg instead of 50 mg per tablet). Overdosing can lead to unintended physiological effects and raises concerns about manufacturing consistency. Since the test was submitted and funded by ironlion-lab, careful evaluation is necessary to assess product consistency across different batches. While Janoshik Analytical is recognized for its transparency and rigorous testing protocols, these findings would benefit from independent validation through third-party testing across multiple samples.
Conclusion
The analysis confirms that anadrol (50mg x 100 tablets/bottle) contains Oxymetholone at 54.26 mg per tablet, which is 8.52% above the labeled claim. While this batch exhibits high purity, the discrepancy in concentration underscores the importance of further testing to ensure accurate dosing across production batches. This report is provided to support educational and harm reduction efforts, helping consumers make informed choices regarding pharmaceutical and peptide products.
Disclaimer
This report is published for educational and harm reduction purposes. Manufacturer-submitted testing may involve inherent biases; however, it can still provide useful data when critically assessed alongside third-party or independent results. Readers are encouraged to use this information responsibly.