A sample of Testosterone Cypionate injectable (250mg/ml), manufactured by PUCA, was submitted and funded by the manufacturer for analytical testing. The lab analysis was conducted by Janoshik Analytical, a trusted third-party laboratory known for performance-enhancing drug verification.
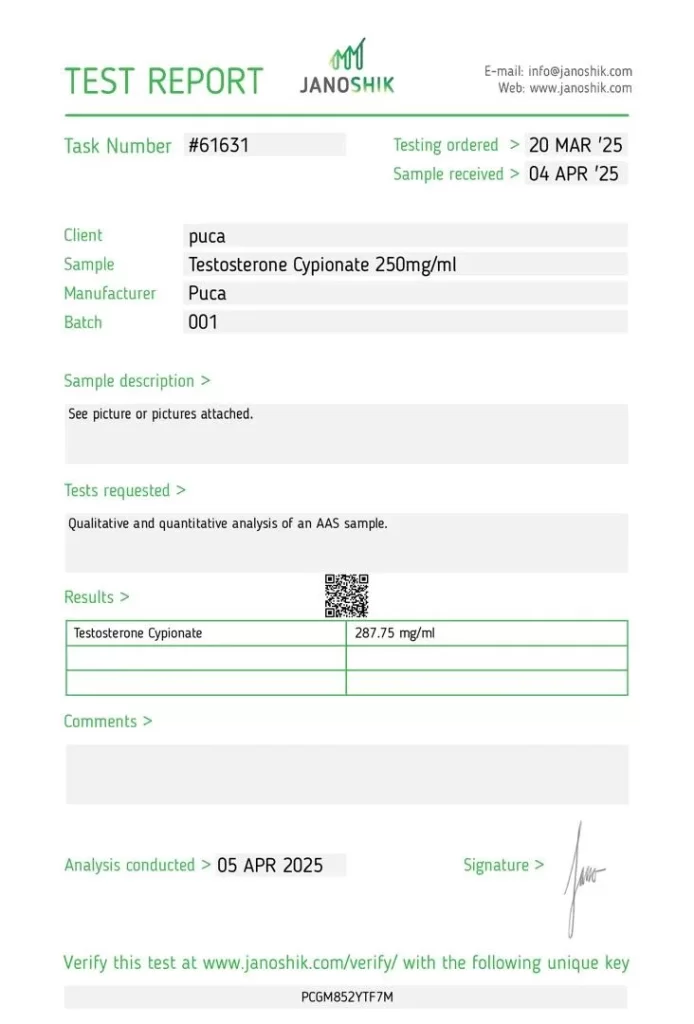
The measured concentration of 287.75 mg/ml reflects a 15.1% overdose relative to the labeled claim of 250 mg/ml. This variance is significant and may affect user dosing accuracy, particularly where titration is critical.
Detailed Report
Product Overview
- Manufacturer: PUCA
- Product Name: Testosterone Cypionate
- Active Ingredient: Testosterone Cypionate
- Labeled Concentration: 250 mg/ml
- Measured Concentration: 287.75 mg/ml
- Batch Number: 001
- Expiration Date: Not Provided
- Delivery Method: Injectable
Sample Acquisition and Testing
- Task Number: #61631
- Testing Ordered: 20 March 2025
- Sample Received: 4 April 2025
- Analysis Conducted By: Janoshik Analytical
- Product Submitted By: PUCA (Manufacturer)
- Analysis Paid For By: PUCA (Manufacturer)
Testing Results
| Component/Test | Specification (Label Claim) | Measured Concentration | Accuracy | Variance |
|---|---|---|---|---|
| Testosterone Cypionate | 250 mg/ml | 287.75 mg/ml | 115.1% | +15.1% |
Verification Details
- Verification URL: https://janoshik.com/tests/61631_PCGM852YTF7M
Evaluation of Manufacturer Testing
The sample showed a substantial overdose, with 287.75 mg/ml measured against the 250 mg/ml label claim — a +15.1% variance. While the presence of the active ingredient is confirmed and the overage may not pose immediate harm to all users, this deviation exceeds typical pharmaceutical tolerance margins.
Because this test was submitted and funded by the manufacturer, it offers insight into PUCA’s internal quality control but lacks the independence of a third-party or consumer-submitted sample. Independent validation is encouraged for broader batch assurance.
Conclusion
This lab analysis of PUCA Testosterone Cypionate 250mg/ml reveals a 15.1% overdose, with 287.75 mg/ml measured. The result highlights the importance of dosage accuracy in injectable hormones and supports further independent oversight, especially for health-critical compounds.
Manufacturer-submitted tests help with transparency, but continued external testing is needed to evaluate consistency across different production runs.
Disclaimer
This report is published for educational and harm reduction purposes only. Manufacturer-funded testing reflects only one batch and may not represent the full product line. For a more comprehensive understanding of quality and dosing reliability, third-party submissions and multi-batch testing are strongly recommended.