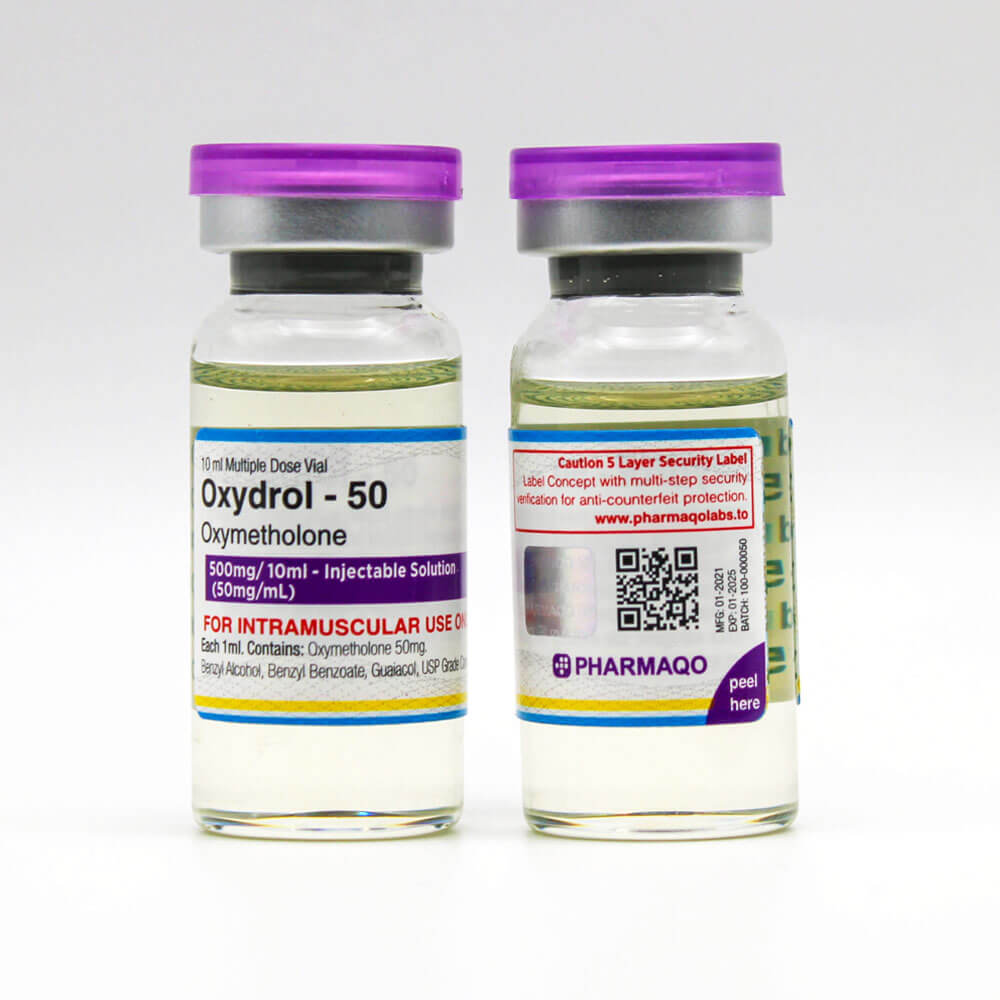
Summary The product Oxydrol 50mg Injection, manufactured by Pharmaqo Labs, underwent independent testing to verify its authenticity and potency. The sample, submitted by Pharmaqo Labs mrp@vitamins.to, was analyzed by Janoshik Analytical, a laboratory specializing in pharmaceutical quality control. The analysis measured the presence of Oxymetholone, with a concentration of 48.32 mg/ml, slightly below the labeled claim of 50 mg/ml by 3.36%. […]