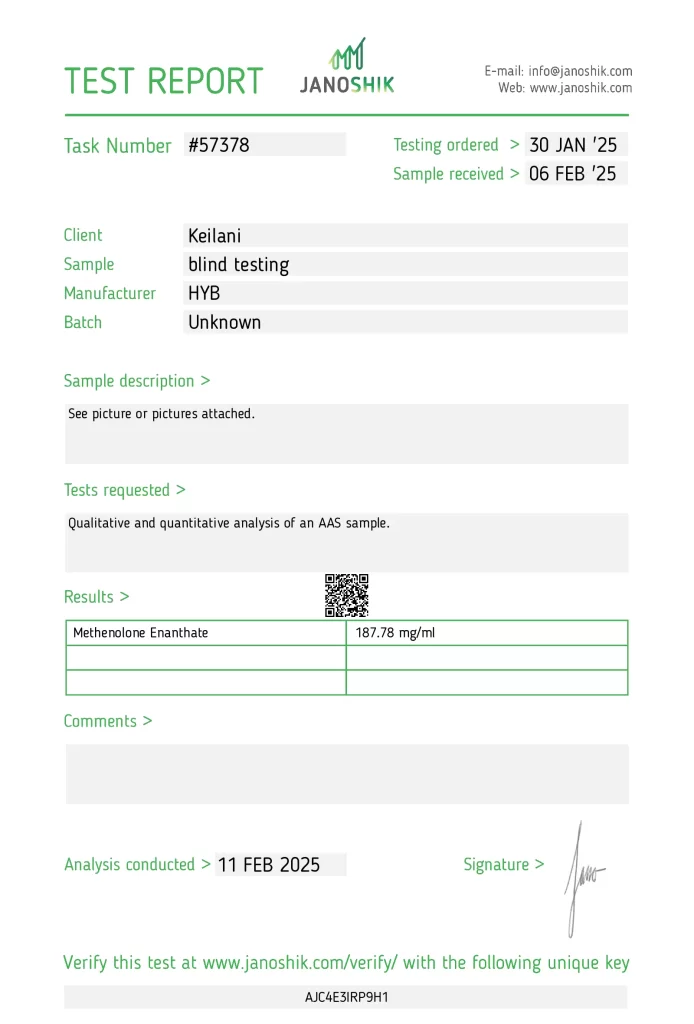
A sample of Methenolone Enanthate (Primobolan) injectable, manufactured by HYB (Yurapeptide), was independently tested to verify its potency. The sample was anonymously procured by an individual consumer, Keilani, and the analysis was paid for by the individual. Testing was conducted by Janoshik Analytical, a laboratory specializing in pharmaceutical quality control.
The measured concentration of 187.78 mg/ml was found against a labeled specification of 200 mg/ml, representing an underdosing of 6.11%. While this is a minor deviation, it falls within the range often considered acceptable in pharmaceutical production. The independent nature of the procurement enhances the credibility of the findings, as it eliminates potential biases associated with manufacturer-funded or reseller-submitted samples.
These results contribute to harm reduction efforts, ensuring transparency and accurate consumer information regarding the quality of HYB’s Methenolone Enanthate.
Detailed Report
Product Overview
- Manufacturer: HYB (Yurapeptide)
- Product Name: Methenolone Enanthate
- Active Ingredient: Methenolone Enanthate
- Batch Number: Unknown
- Expiration Date: Not provided
- Delivery Method: Injectable
Sample Acquisition and Testing
- Task Number: #57378
- Testing Ordered: 30 January 2025
- Sample Received: 6 February 2025
- Analysis Conducted By: Janoshik Analytical
- Product Submitted By: Keilani (Individual)
- Analysis Paid For By: Keilani (Individual)
Testing Results
| Component/Test | Specification (Label Claim) | Measured Concentration | Accuracy | Variance |
|---|---|---|---|---|
| Methenolone Enanthate | 200 mg/ml | 187.78 mg/ml | 93.89% | -6.11% |
Verification Details
- Verification URL: https://janoshik.com/tests/57378_AJC4E3IRP9H1
- Originally Published: https://thinksteroids.com/community/posts/3438150/
Evaluation of Independent Testing
The test results indicate a measured potency of 93.89% relative to the label claim, with a 6.11% variance. While this deviation is relatively minor, it does indicate slight underdosing.
The credibility of this analysis is enhanced by the independent procurement of the sample, which eliminates the risk of selective batch submission by the manufacturer or reseller. Independent consumer-funded testing is one of the most reliable methods for evaluating product consistency across different batches.
Despite the slight underdosing, the findings suggest that HYB’s Methenolone Enanthate contains the stated active ingredient without significant quality concerns. However, continued third-party testing across multiple batches is recommended to ensure long-term consistency in manufacturing.
Conclusion
The analysis of HYB’s Methenolone Enanthate injectable reveals a slight underdosing of 6.11%, with a measured concentration of 187.78 mg/ml versus the 200 mg/ml label claim. While this variation is within an acceptable pharmaceutical tolerance, it highlights the importance of ongoing independent testing to confirm batch-to-batch consistency.
The anonymously procured nature of the sample strengthens the objectivity of this manufacturer-independent evaluation. Consumers are encouraged to refer to third-party testing before making purchasing decisions.
This report serves as an educational resource for harm reduction and informed consumer decision-making.
Disclaimer
This report is published for educational and harm reduction purposes. While individual consumer-funded testing provides valuable insight into product quality, continued independent verification across multiple batches remains essential. Readers are encouraged to interpret this information critically and use independent data for informed decision-making.