Summary
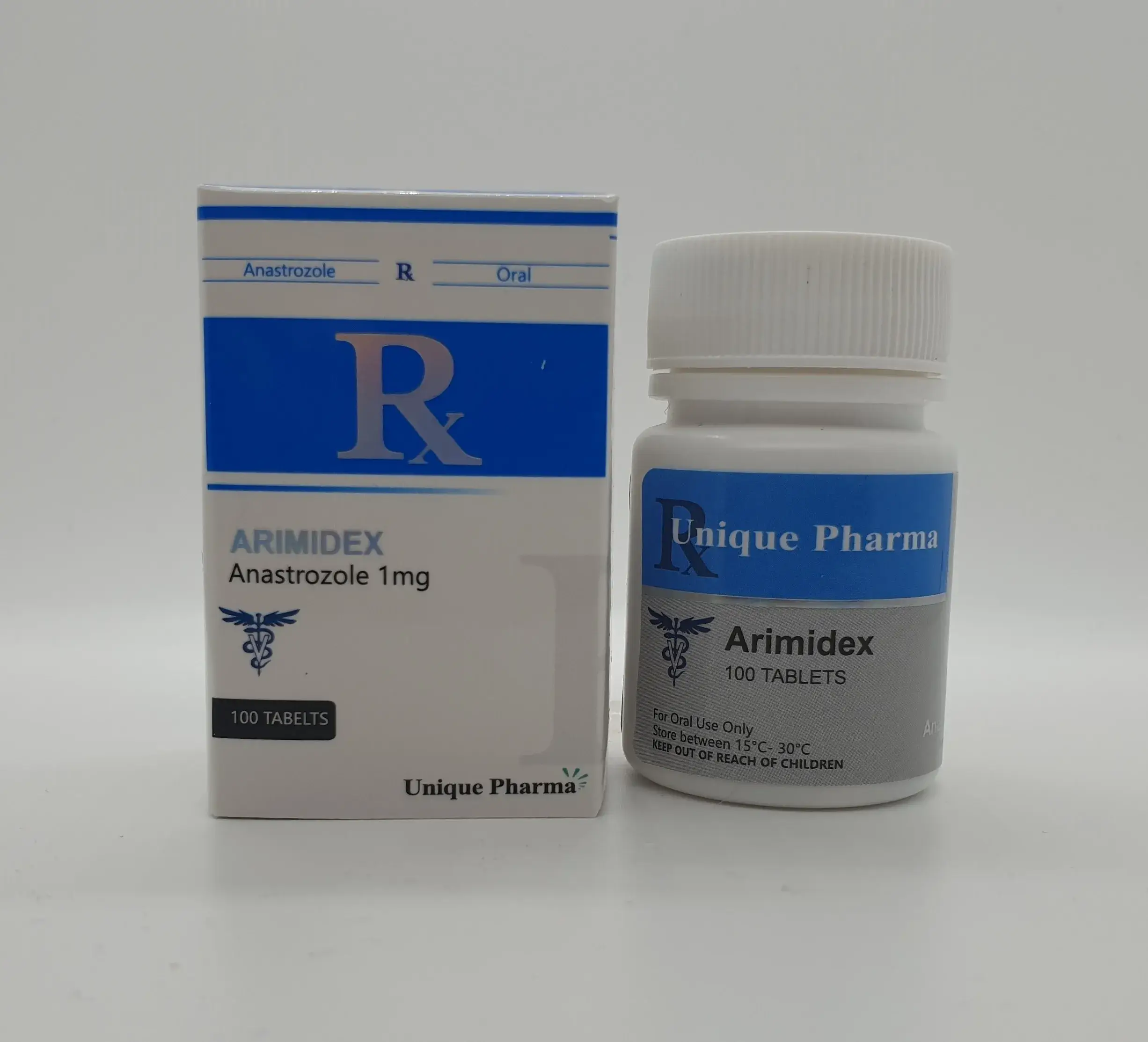
The product Anastrozole 1 mg, manufactured by Unique Pharma, underwent independent testing to verify its authenticity and potency. The sample, identified by batch number GH65721K, was submitted by the reseller Opitropin.EU and analyzed by Janoshik Analytical, a laboratory specializing in pharmaceutical quality control. The analysis revealed that the amount of anastrozole in one pill is 0.82 mg, falling below the labeled claim of 1 mg.
The testing process began on 1 August 2022, with the sample received on 9 August 2022, and the analysis completed on 12 August 2022. The results were verified through retesting twice. While the findings confirm the presence of anastrozole, the lower-than-expected dosage raises questions about the product’s quality and consistency.
Detailed Report
Product Overview
- Manufacturer: Unique Pharma
- Product Name: Anastrozole 1 mg
- Active Ingredient: Anastrozole
- Batch Number: GH65721K
- Delivery Method: Oral tablet
Sample Acquisition and Testing
- Task Number: #22607
- Testing Ordered: 1 August 2022
- Sample Received: 9 August 2022
- Analysis Conducted By: Janoshik Analytical
- Product Submitted By: Opitropin.EU (Reseller)
Testing Results
- Specification: 1 mg per pill (as stated on the label)
- Measured Concentration: 0.82 mg per pill
- Accuracy: 82% of the labeled claim
Verification Details
- Verification URL: https://janoshik.com/tests/22607_QV1KJYFMNP54
- Originally Published: https://thinksteroids.com/community/posts/3118518/
Evaluation of Reseller-Submitted Testing
This analysis highlights the presence of anastrozole but reveals a dosage discrepancy, with the measured concentration falling short of the labeled claim. The submission and funding of the test by the reseller Opitropin.EU warrant careful evaluation, as resellers may select specific batches for testing that may not represent broader market consistency. Independent third-party testing across multiple batches is recommended to ensure product reliability.
Conclusion
The analysis confirms the presence of anastrozole in the tested product, but the measured concentration of 0.82 mg per pill indicates underdosing compared to the labeled claim of 1 mg. While this batch demonstrates some level of quality control, further testing is essential to ensure consistent dosing across the product line. This report is provided to support educational and harm reduction efforts, enabling consumers to make informed decisions about pharmaceutical products.
Disclaimer
This report is published for educational and harm reduction purposes. Reseller-submitted testing may involve inherent biases; however, it can still provide useful data when critically assessed alongside third-party or independent results. Readers are encouraged to use this information responsibly.