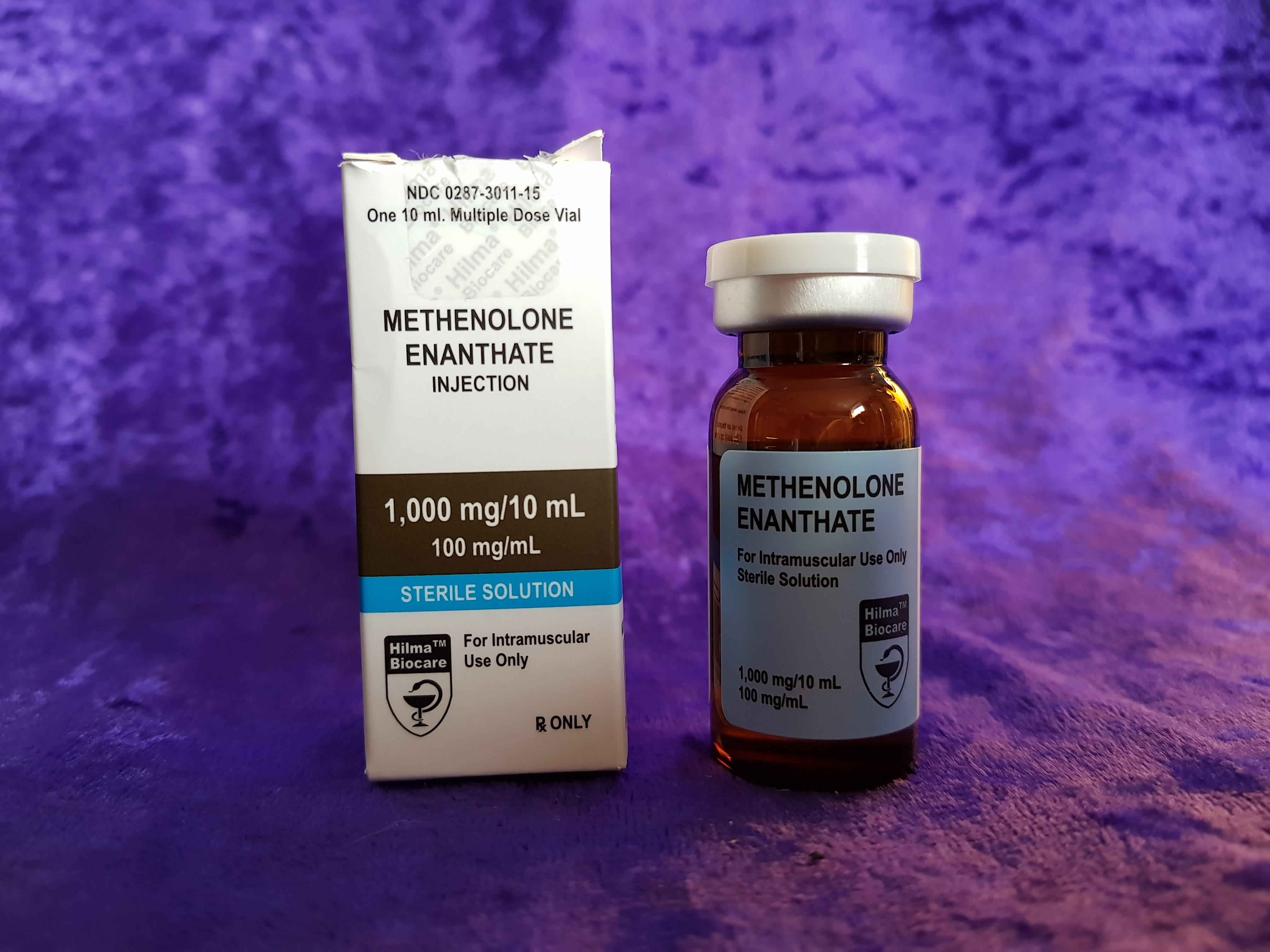
Introduction Hilma BioCare Methenolone Enanthate is presented in a 10-milliliter multidose vial, labeled to contain 100 milligrams of methenolone enanthate per milliliter. To verify the manufacturer’s claims and evaluate its quality, AnabolicLab procured samples of this product from a European-based internet source. These samples were purchased between January 1, 2018, and February 28, 2018, and […]