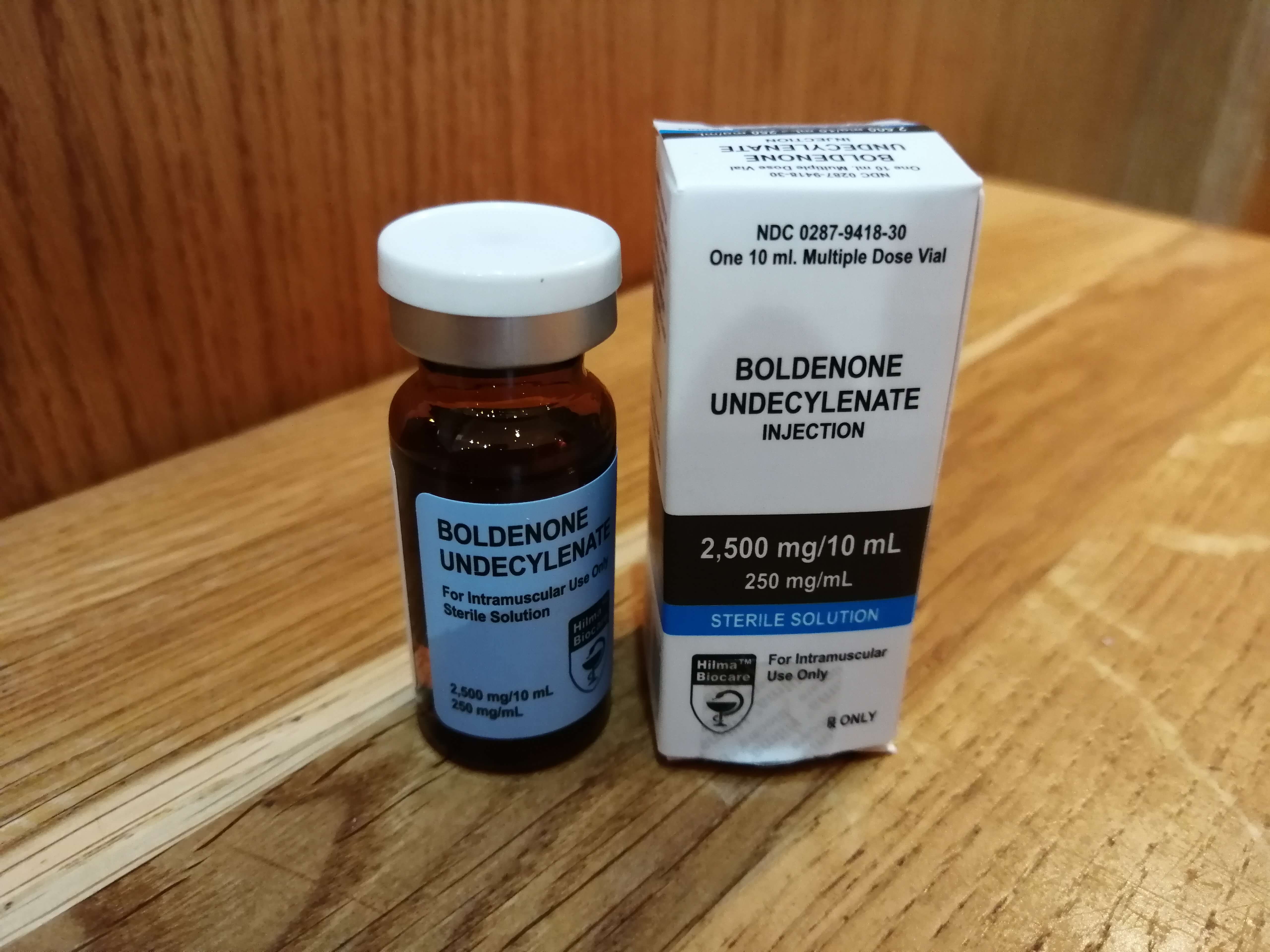
Summary Hilma Biocare Boldenone Undecylenate is marketed as containing 250 mg/ml of boldenone undecylenate and is presented in a 10-milliliter multidose vial. To validate its label claims and ensure product transparency, AnabolicLab procured samples of Boldenone Undecylenate and submitted them for independent laboratory analysis. The analysis, conducted by SIMEC AG, revealed that the product contained […]