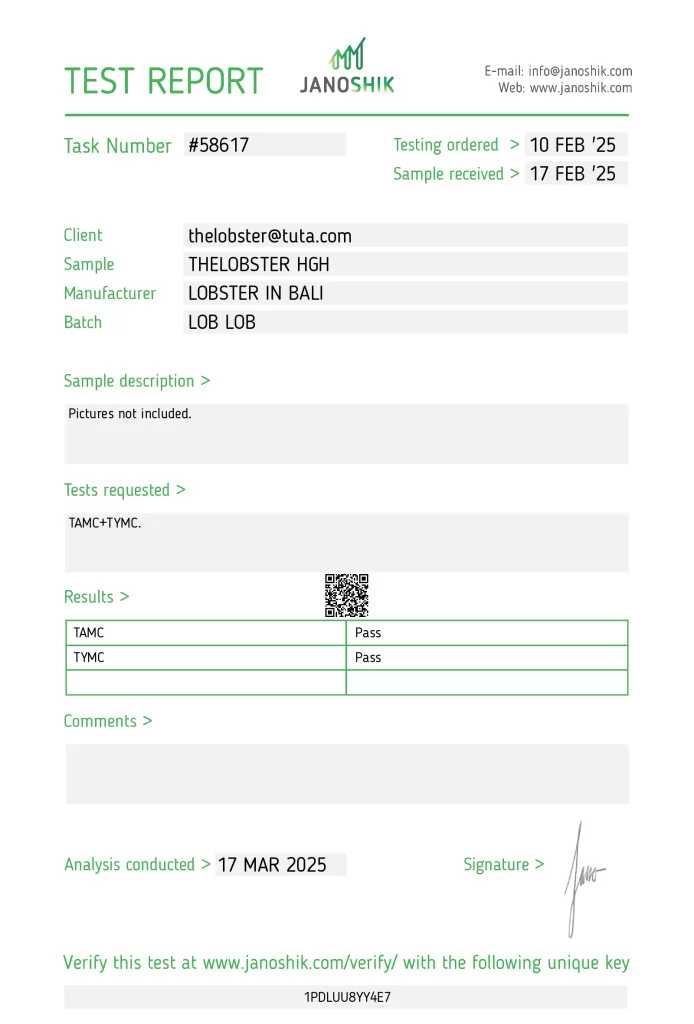
A microbial screening of THELOBSTER HGH, batch LOB LOB, manufactured by LOBSTER IN BALI, was conducted to determine product safety under European Pharmacopoeia standards. The sample was both submitted and paid for by the manufacturer, TheLobster. Testing was performed by Janoshik Analytical, an independent laboratory.
The sample underwent Total Aerobic Microbial Count (TAMC) and Total Yeast and Mold Count (TYMC) tests. Both results returned “Pass”, indicating the sample met microbial safety requirements and was free from unacceptable contamination levels.
Detailed Report
Product Overview
- Manufacturer: LOBSTER IN BALI
- Product Name: THELOBSTER HGH
- Batch Number: LOB LOB
- Testing Type: Microbial Safety (TAMC + TYMC)
- Expiration Date: Not Provided
- Delivery Method: Lyophilized vial
Sample Acquisition and Testing
- Task Number: #58617
- Testing Ordered: 10 February 2025
- Sample Received: 17 February 2025
- Analysis Conducted By: Janoshik Analytical
- Product Submitted By: TheLobster (Manufacturer)
- Analysis Paid For By: TheLobster (Manufacturer)
Testing Results
| Test Type | Result |
|---|---|
| TAMC (Total Aerobic Microbial Count) | Pass |
| TYMC (Total Yeast and Mold Count) | Pass |
Verification Details
- Verification URL: https://janoshik.com/tests/58617_1PDLUU8YY4E7
Evaluation of Manufacturer Testing
The sample successfully passed microbial analysis standards, verifying that THELOBSTER HGH (Batch LOB LOB) is free from harmful microbial contamination. While this affirms the product’s microbiological safety, it does not assess potency, purity, or degradation markers.
Because the test was manufacturer-submitted and funded, it serves as part of internal quality assurance. External validation from independent or consumer-funded sources is still valuable for broader consumer trust.
Conclusion
TheLobster’s HGH sample from Batch LOB LOB passed both TAMC and TYMC tests, confirming its microbiological safety for consumer use. These findings support proper sterile handling and production processes, at least for this tested lot.
Disclaimer
This report is shared for educational and harm reduction purposes. Microbial testing alone does not reflect potency or formulation quality. Results are based on a single, manufacturer-submitted sample. Broader evaluation of product consistency should include ongoing, third-party potency and purity testing.