Summary
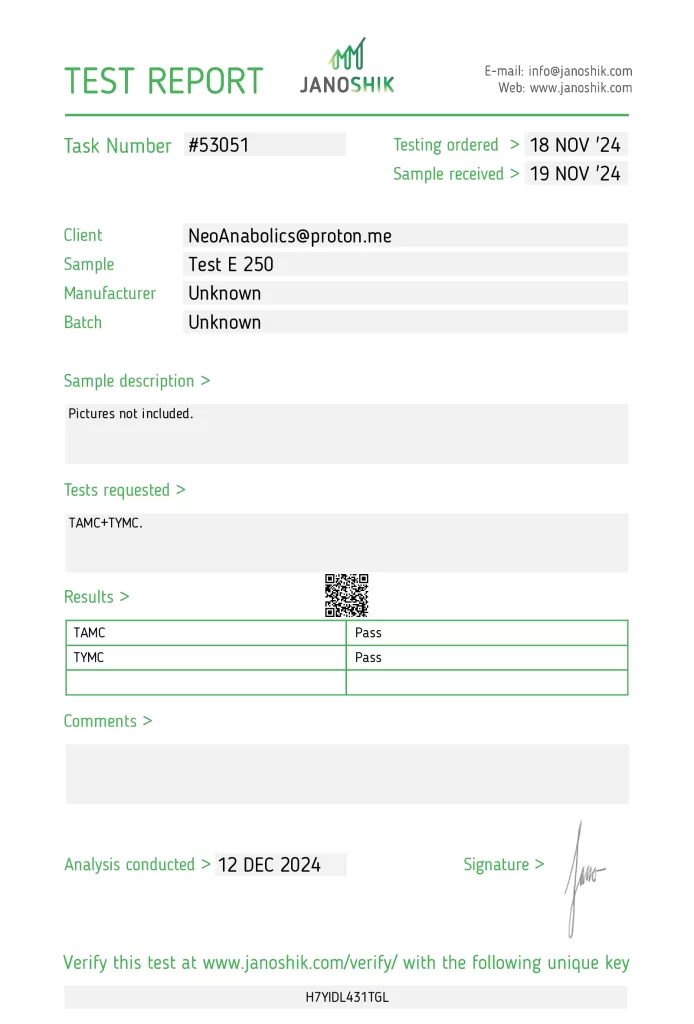
The product Test E 250, manufactured by NeoAnabolics, underwent independent testing to verify its microbiological safety. The sample was submitted by NeoAnabolics and analyzed by Janoshik Analytical, a laboratory specializing in pharmaceutical quality control. The analysis assessed Total Aerobic Microbial Count (TAMC) and Total Yeast and Mold Count (TYMC), both of which passed the required standards.
The testing process began on 18 November 2024, with the sample received on 19 November 2024, and analysis completed on 12 December 2024. The results confirm that the product meets microbiological safety standards, but independent third-party testing remains essential for comprehensive quality assurance. This report serves as an educational resource to promote harm reduction and informed decision-making.
Detailed Report
Product Overview
- Manufacturer: NeoAnabolics
- Product Name: Test E 250
- Active Ingredient: Not specified in the report
- Batch Number: Unknown
- Expiration Date: Not provided
- Delivery Method: Injectable
Sample Acquisition and Testing
- Task Number: #53051
- Testing Ordered: 18 November 2024
- Sample Received: 19 November 2024
- Analysis Conducted By: Janoshik Analytical
- Product Submitted By: NeoAnabolics (Manufacturer)
- Analysis Paid For By: NeoAnabolics (Manufacturer)
Testing Results
- TAMC (Total Aerobic Microbial Count): Pass
- TYMC (Total Yeast and Mold Count): Pass
Verification Details
Verification URL: https://janoshik.com/tests/53051_H7YIDL431TGL
Evaluation of Manufacturer-Submitted Testing
This microbiological analysis confirms the product meets cleanliness and safety standards, but additional testing is recommended to ensure consistency across different batches. Since NeoAnabolics submitted and funded the test, third-party validation is encouraged to maintain unbiased quality assessment. While Janoshik Analytical is known for its strict testing protocols, external verification would enhance product reliability.
Conclusion
The analysis confirms that Test E 250 meets microbiological safety requirements, as both TAMC and TYMC tests passed. Although this indicates a clean product free from microbial contamination, further analysis should be conducted for potency and purity validation. This report aims to support educational and harm reduction efforts, assisting consumers in making informed decisions regarding injectable products.
Disclaimer
This report is published for educational and harm reduction purposes. Manufacturer-submitted testing may involve inherent biases; however, it still provides useful data when critically assessed alongside third-party results. Readers are encouraged to use this information responsibly.