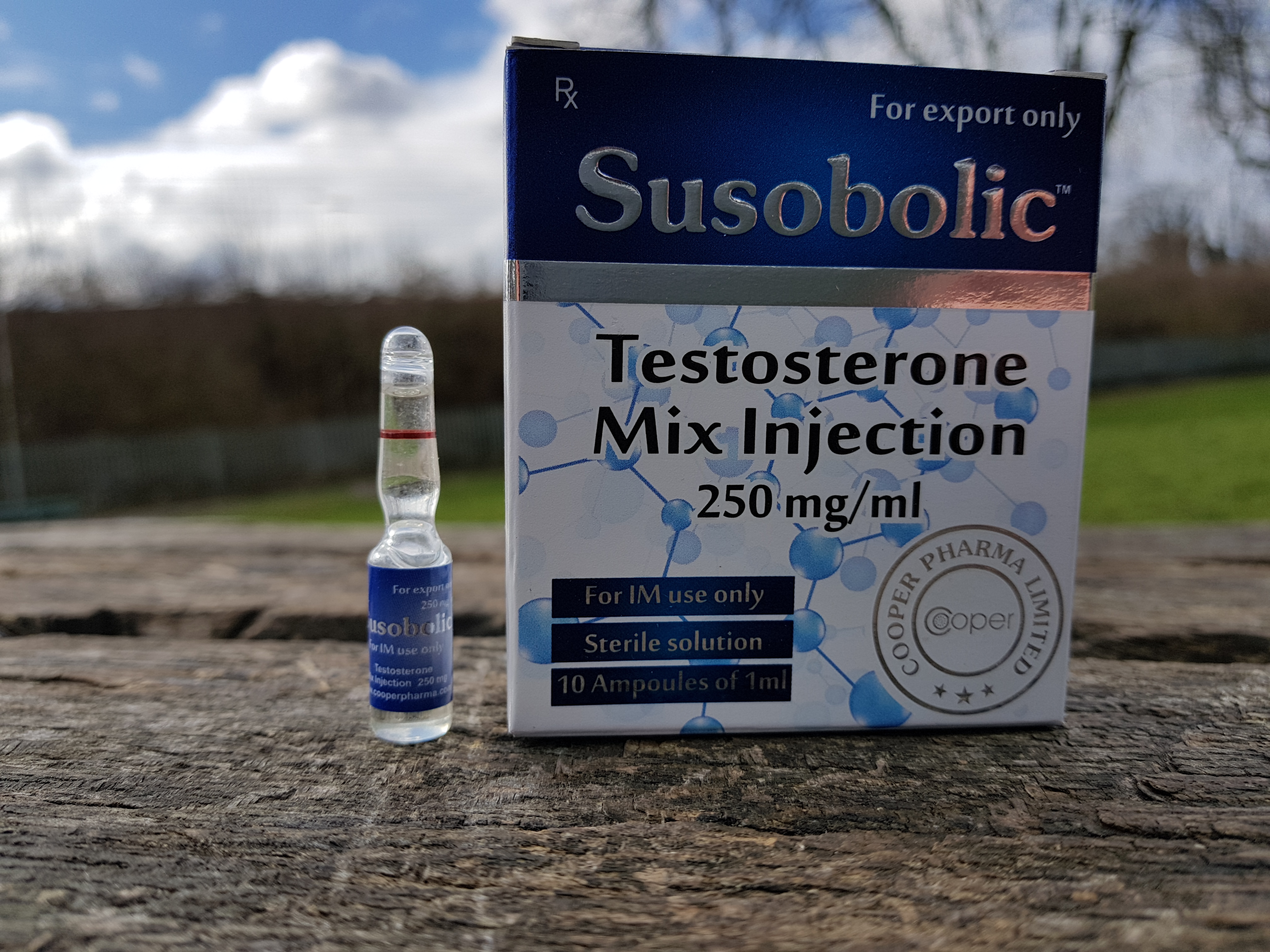
Summary Cooper Pharma Susobolic, presented in an injectable form with a specified blend of four testosterone esters, underwent independent testing to validate its composition and dosage accuracy. The sample, marked with batch number LE 1327 (manufactured January 2015, expiration December 2018), was submitted and funded by AnabolicLab.com. Analysis was performed by SIMEC AG, a highly […]